Abstract
Background Optimal treatment for patients with relapsed/refractory (R/R) acute myeloid leukemia (AML), particularly after molecular relapse or disease recurrence following allogeneic hematopoietic stem cell transplantation (HSCT), is undefined. Intensive salvage chemotherapy is frequently offered but is often ineffective and carries significant risk of toxicity, especially post-HSCT. Such toxicity is particularly undesirable in those patients with low burden disease, such as molecular relapse. While venetoclax (VEN, BCL-2 inhibitor) combined with FLAG-Ida was effective in a phase Ib/2 study of R/R AML (DiNardo, 2021), molecular relapse was not included, and only 14/68 subjects were treated at post-HSCT relapse. Concerns regarding toxicity of that regimen persist. Alternatively, VEN combined with hypomethylating agents or low dose cytarabine (HMA, LDAC) has shown clinical activity with good tolerability in relapsed AML, but only in small studies with little focus on outcomes following molecular or post-transplant relapse. Herein, we report outcomes after non-intensive VEN salvage combinations in a large population of patients with R/R AML treated in the UK.
Methods The outcomes of adults with R/R AML receiving non-intensive VEN combinations as salvage therapy in UK hospitals were studied. Patients included had a diagnosis of AML/high risk myelodysplastic syndrome (MDS, ≥10% bone marrow blasts); refractory disease/relapse following ≥1 line of chemotherapy and at least 3 months follow up from initiation of VEN combined with non-intensive chemotherapy regimens. Response was defined by European Leukemia Net (ELN) criteria.
Results 126 evaluable patients (117 AML, 9 high risk MDS) were treated during 2017-2022 (Table 1). The median age was 58 years (range 17-83 years). By ELN 2017 criteria, 32 AML patients were adverse risk, 39 intermediate and 42 favourable (4 unknown). Diagnostic analyses, where available, demonstrated mutations of NPM1 in 34 AML patients, IDH1/2 (n=17), tumour suppressor genes (n=12) and signalling pathway genes (n=37), predominantly FLT3 (n=21). 30 patients had primary refractory disease whilst 96 had relapsed (45 pre-HSCT, 51 post-HSCT). 19 patients had molecular measurable residual disease (MRD; 18 molecular relapse, 1 molecular persistence) whilst 103 had morphologic, flow cytometric or extramedullary disease (4 unknown). VEN was combined with HMA (n=75), LDAC (n=44) or used with other low intensity agents/as monotherapy (n=7).
For the whole cohort, with a median follow up of 16.6 months, the median overall survival (OS) was 8.5 months (95% confidence interval [CI] 4.8-12.2 months), including when censored at subsequent transplant. 56 (44%) patients achieved CR/CRi; best response occurred after a median of 1 cycle (range 1-6).
Those patients with ELN 2017 favourable risk disease experienced the best outcomes (median OS not reached vs 6.5 months [95% CI 4-9-8.2 months] for the remaining cohort; p <0.001). Median OS was inferior for both intermediate and adverse risk groups at 7.1 months (95% CI 4.5-9.7 months) and 5.8 months (95% CI 3.6-8.1 months), respectively.
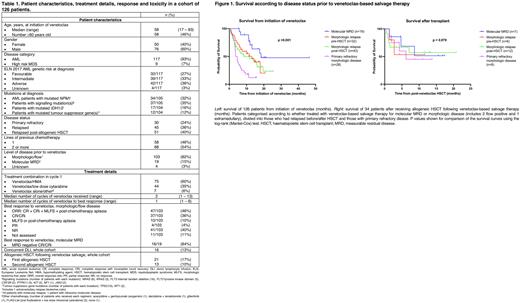
Notably, 16/19 (84%) patients treated for molecular MRD achieved molecular CR/CRi. This group experienced superior median OS compared with the 103 patients with morphologic/flow positive/extramedullary disease (18.4 months vs 7.1 months, 95% CI 4.9-9.3 months; p = 0.004), Figure 1.
In total, 34 patients (27%) were successfully bridged to transplant after VEN salvage. Median OS from transplant not reached, despite follow up of 11.8 months, with similar outcomes regardless of whether disease was R/R when treated with VEN combinations (Figure 1). Of 51 patients treated for post-HSCT relapse, 21 (41%) achieved CR/CRi, 16 (31%) received donor lymphocyte infusions and 13 (25%) proceeded to second HSCT.
Toxicity of non-intensive VEN combinations was consistent with previous reports: tumour lysis syndrome was reported in 6 (5%) patients and infection requiring antibiotics in 76 (60%).
Conclusion These real-world data demonstrate that VEN-based non-intensive combinations are well tolerated and active in R/R AML, particularly in patients with molecular relapse, and represent an important route to potentially curative cellular therapy.
Disclosures
Wood:Cellectis: Research Funding; Gilead: Other: Partner is a current employee. Kulasekararaj:Ra Pharma: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sobi: Consultancy, Honoraria, Speakers Bureau; Samsung: Consultancy, Honoraria, Speakers Bureau; F. Hoffmann-La Roche: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Biocryst: Consultancy, Honoraria, Speakers Bureau; Apellis: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria, Speakers Bureau; Akari: Consultancy, Honoraria, Speakers Bureau; Achillion: Consultancy, Honoraria, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Speakers Bureau. Taussig:Abbvie: Other: Funding to attend ASH annual meeting & exposition. Knapper:Astellas: Speakers Bureau; BMS: Consultancy; Jazz: Consultancy, Honoraria, Speakers Bureau; Novartis: Research Funding, Speakers Bureau; Servier: Consultancy, Honoraria. Dillon:Astellas: Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; Amgen: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; Shattuck: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees; AvenCell: Membership on an entity's Board of Directors or advisory committees. Murthy:Jazz: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: Travel grant; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau. Mehta:Astellas: Honoraria, Speakers Bureau; Jazz: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau. Khan:Abbvie: Honoraria; Astellas: Speakers Bureau; TC BioPharm: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. O'Nions:Ellipses Pharma: Research Funding; Astellas: Honoraria. Craddock:Novartis: Consultancy; Celgene: Consultancy, Research Funding; JAZZ: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Daiichi-Sankyo: Consultancy. Copland:Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cyclacel Ltd: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Krishnamurthy:Gilead: Consultancy, Honoraria; Astellas: Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Honoraria.
OffLabel Disclosure:
The FDA has approved venetoclax in combination with azacitidine or decitabine or low-dose cytarabine for the treatment of newly-diagnosed acute myeloid leukemia (AML) in adults who are age 75 years or older, or who have comorbidities that preclude use of intensive induction chemotherapy. Here we report on the use of venetoclax-based non-intensive combinations in treatment of relapsed/refractory AML.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal